Acids are sour in taste and are present in all our food. Acids and base are present in almost all foods we have. Acids are sour in taste and change the colour of litmus from blue to red. Bases are bitter and change the colour of the litmus from red to blue.
How Acids and Bases React With Metals
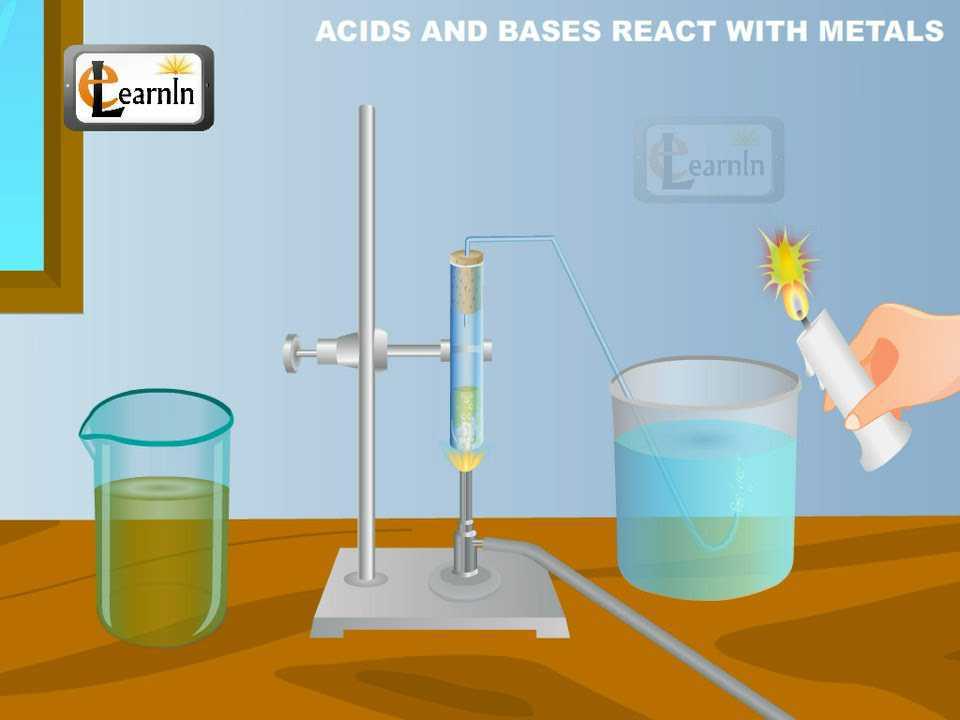
The metal in the above reactions displaces hydrogen from the acids. This is seen as hydrogen gas. The metal combines with the remaining part of the acid and forms a compound called a salt. Thus,
The reaction of a metal with an acid can be summarized as –
Acid + Metal = Salt + Hydrogen gas
How are Acids and Base Common
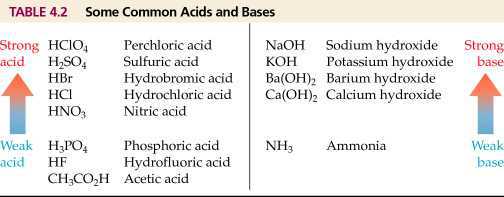
We have seen that all acids have similar chemical properties. What leads to this similarity in properties? We saw that all acids generate hydrogen gas on reacting with metals, so hydrogen seems to be common to all acids. Let us perform an Activity to investigate whether all compounds containing hydrogen are acidic.
The bulb will start glowing in the case of acids. But you will observe that glucose and alcohol solutions do not conduct electricity. The glowing of the bulb indicates that there is a flow of electric current through the solution. The electric current is carried through the solution by ions. Since the cation present in acids is H+, this suggests that acids produce hydrogen ions, H+ (aq), in solution, which is responsible for their acidic properties.
Acids Which Dissolve in Water
The process of dissolving an acid or a base in water is a highly exothermic one. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating. Look out for the warning sign on the can of concentrated sulphuric acid and on the bottle of sodium hydroxide pellets.
Mixing an acid or base with water results in the decrease in the concentration of ions (H3O+/OH–) per unit volume. Such a process is called dilution and the acid or the base is said to be diluted.
How Strong are Acid or Base Solutions?
Acid-base indicators can be used to distinguish between an acid and a base. We have also learned in the previous section about dilution and decrease in concentration of H+ or OH– ions in solutions. We can do this by making use of a universal indicator, which is a mixture of several indicators. The universal indicator shows different colours at different concentrations of hydrogen ions in a solution. A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale, we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value. The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH– ion concentration in the solution, that is, increase in the strength of alkali. Generally, paper impregnated with the universal indicator is used for measuring pH.
Recap
- Acid-base indicators are dyes or mixtures of dyes which are used to indicate the presence of acids and bases.
- Acidic nature of a substance is due to the formation of H+(aq) ions in solution. Formation of OH–(aq) ions in solution are responsible for the basic nature of a substance.
- When an acid reacts with a metal, hydrogen gas is evolved and a corresponding salt is formed.
- When a base reacts with a metal, along with the evolution of hydrogen gas a salt is formed which a negative ion has composed of the metal and oxygen.
- When an acid reacts with a metal carbonate or metal hydrogen carbonate, it gives the corresponding salt, carbon dioxide gas and water.
- Acidic and basic solutions in water conduct electricity because they produce hydrogen and hydroxide ions respectively.
- The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
- A neutral solution has a pH of exactly 7, while an acidic solution has a pH less than 7 and a basic solution a pH more than 7.
- Living beings carry out their metabolic activities within an optimal pH range.
- Mixing concentrated acids or bases with water is a highly exothermic process.
- Acids and bases neutralize each other to form corresponding salts and water.
- The water of crystallization is the fixed number of water molecules chemically attached to each formula unit of a salt in its crystalline form.
- Salts have various uses in everyday life and in industries.





























Comments