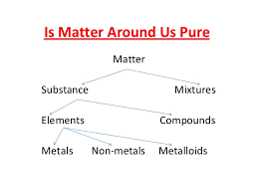
Most of the matter around us is not pure. Either they have impurities or they exist as a mixture of two components. A pure substance always has a single type of particle, of two types it is a pure single form of matter.
Mixture
Mixtures are constituted of more than one pure form of substances. A substance cannot be separated into other forms of matter by any physical means. They can be distinguished into two types:
Heterogeneous Mixtures
They do not have equal composition throughout the solution.
Examples:
- Ice cubes in a drink

- Sand and water

- Oil and salt

2. Homogeneous Mixture
They have the same composition throughout the mixture.
- Water

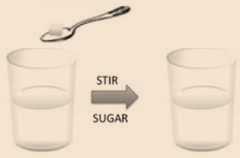
- Sugar and water
- White vinegar

Solution
A solution is basically a homogenous mixture of two or more pure substances. A solution consists of a solvent and a solute as its components. The component of the solution that dissolves the other component in it is called the solvent. The component in the solution that is dissolved in the solvent (usually present in lesser quantity) is called the solute.
Examples:
- A solution of sugar in water is solid in liquid solution. In this solution, sugar is the solute and water is the solvent.
- A solution of iodine in alcohol known as ‘tincture of iodine’, has iodine (solid) as the solute and alcohol (liquid) as the solvent.
- Aerated drinks like soda water etc., are gas in liquid solutions. These contain carbon dioxide (gas) as solute and water (liquid) as a solvent.
- Air is a mixture of gas in gas. Air is a homogeneous mixture of a number of gases. Its two main constituents are oxygen (21%) and nitrogen (78%). The other gases are present in very small quantities
Properties of a Solution
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9 metre) in diameter. So,they cannot be seen by naked eyes.
- Because of very small particle size, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- The solute particles cannot be separated from the mixture by the process of filtration. The solute particles do not settle down when left undisturbed, that is, a solution is stable.
Suspension
A non-homogenous solution in which solids are dispersed in the liquid and the solid particles are relatively big in size is called a suspension.
Properties of a Suspension:
- It is a heterogeneous mixture.
- The particles of a suspension can be seen by the naked eye.
- The particles of a suspension scatter a beam of light passing through it and make its path visible.
- The solute particles settle down when a suspension is left undisturbed, that is, a suspension is unstable. They can be separated from the mixture by the process of filtration. When the particles settle down, the suspension breaks and it does not scatter light anymore.
Colloids
The particles of a colloid are uniformly spread throughout the solution. Due to the relatively smaller size of particles, as compared to that of a suspension, the mixture appears to be homogeneous. But actually, a colloidal solution is a heterogeneous mixture.
Properties of a Colloid
- A colloid is a heterogeneous mixture.
- The size of particles of a colloid is too small to be individually seen by naked eyes.
- Colloids are big enough to scatter a beam of light passing through it and make its path visible.
- They do not settle down when left undisturbed, that is, a colloid is quite stable
Separation of Materials
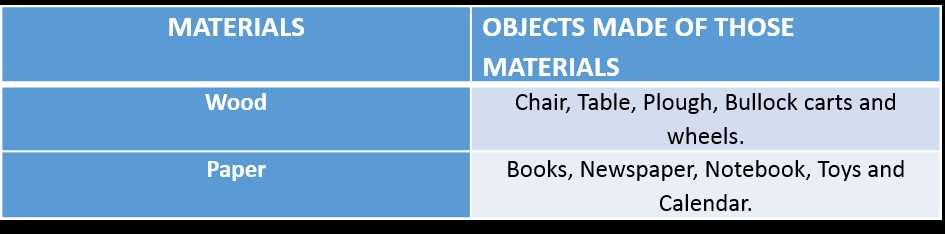
All objects around us are made of some materials. These materials may be classified as glass, metal, plastics, wood, cotton, paper or mud. So, each of these objects are classified based on the materials they are made of.
Table 1
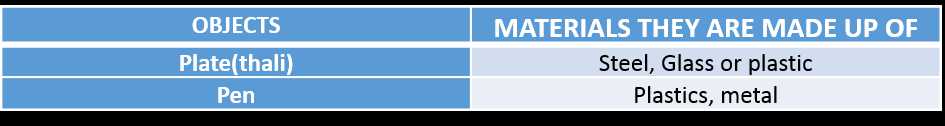
Table 2
Classification Based On Properties Of Materials
Appearance
Materials usually look different from each other. Wood looks very different from iron. Iron appears different from copper or aluminum. At the same time, there may be some similarities between iron, copper and aluminum that are not there in wood.
Hardness
When we press different materials with your hands, some of them may be hard to compress while others are easily compressible. Then, take a metal key and try to scratch with it, the surface of a piece of wood, aluminum, a piece of stone, a nail, candle, chalk and any other material. You can easily scratch some materials, while some cannot be scratched so easily. Materials which can be compressed or scratched easily are called soft while some other materials which are difficult to compress are called hard. For example, cotton or sponge is soft while the iron is hard. In appearance, materials can have different properties, like luster, hardness, be rough or smooth.
Transparency
Those substances or materials, through which things can be seen, are called transparent. Glass, water, air and some plastics are examples of transparent materials.
Shopkeepers usually prefer to keep biscuits, sweets and other eatables in transparent containers of glass or plastic, so that buyers can easily see these items
On the other hand, there are some materials through which you are not able to see. These materials are called opaque. You cannot tell what is kept in a closed wooden box, a cardboard carton or a metal container. Wood, cardboard and metals, are examples of opaque materials.The materials through which objects can be seen, but not clearly, are known as translucent.
Recap
- Heterogeneous mixtures do not have equal composition throughout the solution.
- Homogenous mixtures have equal composition throughout the solution.
- A suspension is a heterogeneous mixture which has solid dispersed in the liquid.
- A colloid is a heterogeneous mixture which appears like a homogenous solution.
- Cotton or sponge is soft while the iron is hard. In appearance, materials can have different properties, like luster, hardness, be rough or smooth.
- Those substances or materials, through which things can be seen, are called transparent. Glass, water, air and some plastics are examples of transparent materials.
- Handpicking can be used for separating slightly larger sized impurities like the pieces of dirt, stone, and husk from wheat, rice or pulses.



























Comments