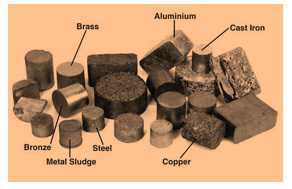
Metals
- Metals are defined as those materials which have the characteristics of being shiny, hard, ductile, fusible, malleable, etc.
- Examples are iron, silver, gold, copper, aluminum, etc.
Physical properties of metals
Malleability
- This property of metals allows it to get beaten into thin sheets.
- It is used in the silver foils which are used to decorate sweets and aluminium foils which are used to wrap food.

Conductivity
- Current and heat pass through the metals easily by conductivity.
- Examples are nail, copper, iron rod, etc.

Ductility
- This property makes the metals drawn into the wires.

Sonorous
- When metals are hit they produce ringing sounds due to this property.

Lustrous
- When metals shine and are sufficient to reflect incident light, it becomes possible due to the lustrous property of metals.

Non - Metals
- When metals do not possess such characteristics of metals then they are termed as Non-Metals.
- Nonmetals are poor conductors of electricity and heat and are non-sonorous.
- Examples are carbon, oxygen, sulphur, etc.

Chemical Properties for metals and nonmetals
- Reaction with oxygen
- For Metals
When oxygen and metals react, metallic oxides are formed.
- For non-metals
Oxides are produced when non-metals react with oxygen
2. Reaction with water
- For metals
Line in case of sodium, some metals with water react vigorously. But some metals react slowly like in the case of iron.
- For non-metals
Most of the non-metals do not react with water but still, there are some non-metals which react a little in the air like phosphorus. It is usually kept in water to prevent an explosion.
Application of metals
- These are generally used in making automobiles, machines, cars, aeroplanes, satellites, etc.
- Some metals are also used to make wires like copper, etc.
- Ornaments like silver, gold, etc. are also made from metals.
Application of non-metals
- A very important non-metal is oxygen which is necessary for the survival of all the living beings.
- Nonmetals are also used as fertilizers to induce the growth of plants.
- Nonmetals are used as antiseptic.
- Crackers are made up of nonmetals.
Recap
- Metals are defined as those materials which have the characteristics of being shiny, hard, ductile, fusible, malleable, etc.
- Examples are iron, silver, gold, copper, aluminium, etc.
- When metals do not possess such characteristics of metals then they are termed as Non-Metals.
- Nonmetals are poor conductors of electricity and heat and are non-sonorous.
- Examples are carbon, oxygen, sulphur, etc.


































Comments