With time, new elements began to be discovered. And scientists started to discover that there is something periodic about the properties of these elements and their mass number of these elements. And so they started designing periodic tables which helped in the periodic classification of elements and was a huge invention in the field of elements.
Different Old Periodic Tables for Classification of Elements
Dobereiner’s Law of Triads
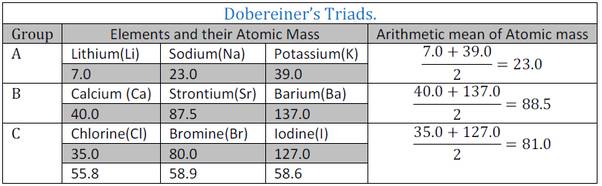
In the year 1817, Johann Wolfgang Döbereiner, a German chemist, tried to arrange the elements with similar properties into groups. He identified some groups having three elements each. So he called these groups ‘triads’. Döbereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
Newland’s Law Of Octave
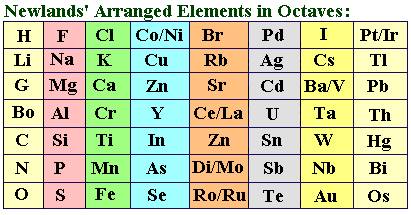
The attempts of Döbereiner encouraged other chemists to correlate the properties of elements with their atomic masses. In 1866, John Newlands, an English scientist, arranged the then known elements in the order of increasing atomic masses. He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element. He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music. Therefore, he called it the ‘Law of Octaves’. It is known as ‘Newlands’ Law of Octaves’. In Newlands’ Octaves, the properties of lithium and sodium were found to be the same. Sodium is the eighth element after lithium. Similarly, beryllium and magnesium resemble each other.
Mendeleev’s Periodic Table
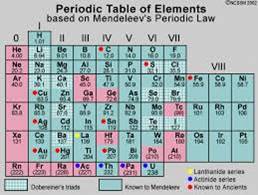
When Mendeleev started his work, 63 elements were known. He examined the relationship between the atomic masses of the elements and their physical and chemical properties. Among chemical properties, Mendeleev concentrated on the compounds formed by elements with oxygen and hydrogen. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. The formulae of the hydrides and oxides formed by an element were treated as one of the basic properties of an element for its classification. He then took 63 cards and on each card he wrote down the properties of one element. He sorted out the elements with similar properties and pinned the cards together on a wall. He observed that most of the elements got a place in a Periodic Table and were arranged in the order of their increasing atomic masses. It was also observed that there occurs a periodic recurrence of elements with similar physical and chemical properties. On this basis, Mendeleev formulated a Periodic Law, which states that ‘the properties of elements are the periodic function of their atomic masses’.
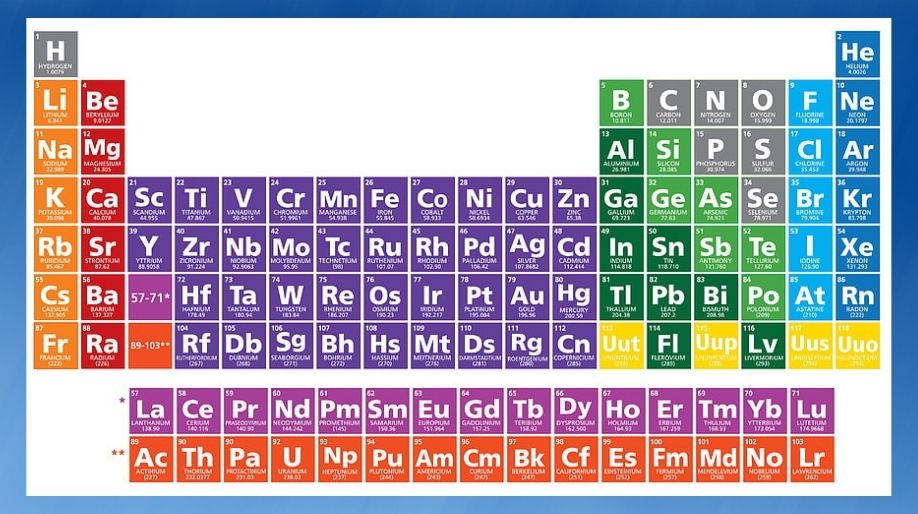
Modern Periodic Table

Recap
- Elements are classified on the basis of similarities in their properties.
- Döbereiner grouped the elements into triads and Newlands gave the Law of Octaves.
- Mendeleev arranged the elements in increasing order of their atomic masses and according to their chemical properties.
- Mendeleev even predicted the existence of some yet to be discovered elements on the basis of gaps in his Periodic Table.
- Anomalies in the arrangement of elements based on increasing atomic mass could be removed when the elements were arranged in order of increasing atomic number, a fundamental property of the element discovered by Moseley.
- Elements in the Modern Periodic Table are arranged in 18 vertical columns called groups and 7 horizontal rows called periods.
- Elements thus arranged show periodicity of properties including atomic size, valency or combining capacity and metallic and non-metallic character.




























Comments