The whole world is surrounded by matter. Matter is anything that occupies spaces, has some mass and can be perceived by senses. Matter can neither be created nor destroyed.
Three States of Matter
There are three states of matter. They are:
All of them occupy a definite amount of space, has some mass and can be perceived by the senses. So, they qualify as matter.
|
SOLIDS
|
LIQUIDS |
GASES |
|
They have a definite mass, definite shape and definite shape. |
They have a definite mass, definite shape but no definite shape. |
They have a definite mass but no definite shape and no definite shape. |
|
They are rigid, cannot flow. |
They are less rigid and can flow. |
They are not rigid and flow easily. |
|
They cannot be compressed. |
They are slightly compressible. |
They are highly compressible. |
|
These particles do not diffuse. |
These particles may diffuse. |
These particles diffuse rapidly. |
|
They have minimum intermolecular space. |
They have more intermolecular space. |
They have very large intermolecular space. |
|
The attraction between particles here is maximum. |
The attraction between particles here is less than solids. |
The attraction between particles here is minimum. |
|
The motion of particles here is minimum. |
The motion of particles here is more than solids. |
The motion of particles here is maximum. |
|
Ex: 1) Paper 2)Wood |
Ex: 1) Water 2)Alcohol |
Ex: 1) Hydrogen 2) Carbon Dioxide |
Demonstrations to Study States of Matter
- To show that solids have a definite volume and shape whereas liquid has a definite volume but no definite shape.
Experiment:
- Place metal rods in different shaped containers.
- Add equal amounts of water slowly into each container by means of a tumbler.
- The rod does not change its volume or shape.
- The water does not change the volume but it takes up the shape of the container in each case.
Conclusion: Solids have a definite volume and shape whereas liquid has a definite volume but no definite shape.
2)To show that gases have no definite volume or shape.
Experiment:
- Take a gas jar filled with a coloured gas (Ex. Nitrogen Dioxide)
- Hold the jar inverted over a beaker.
- The coloured gas enters the beaker and completely fills it taking up the volume and shape of the beaker.
- The coloured gas enters the beaker and completely fills it taking up the volume and shape of the beaker.
Conclusion: Gases have no definite volume or shape.
3)To show that Solids are not compressible while gases are highly compressible.
Experiment:
- Take a solid block of wood and press it. It does not compress.
- Take two plastic bottles one filled with water and the other empty (gas).
- The bottle with water is squeezed slightly whereas empty bottle is squeezed a lot more.
Conclusion: Solids are not compressible while gases are highly compressible.
4)To show molecules of a solid attract each other
Experiment:
- Place globules of mercury in a petri dish at a distance apart.
- Shake the dish slowly. The molecule globules come together forming one big globule of mercury.
Conclusion: The above demonstration concludes that there is a force of attraction between particles of matter.
5) To show molecules of a substance are in a constant motion.
Experiment:
- Add a crystal of potassium permanganate to water. The water completely turns purple.
- Open a bottle of perfume. The smell can be perceived even at a distance.
- Add talcum powder to water kept in a petri dish and observe the movement of the particles through a microscope. The particles are in a random motion.
Conclusion: Molecules of a substance are in a constant motion.
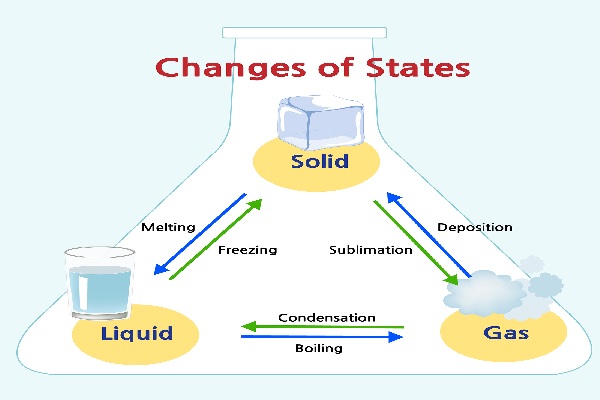
Interconversion of Matter
Interconversion of matter involves a change in state of matter from one state to another and back to the original state. This change is generally brought about by factors like temperature and pressure.
Recap
- The matter is anything that occupies spaces, has some mass and can be perceived by senses.
- There are three states of matter. They are solids, liquids, gases.
- Solids have a definite volume and shape whereas liquid has a definite volume but no definite shape.
- Gases have no definite volume or shape.
- Solids are not compressible while gases are highly compressible.
- Molecules of a solid attract each other.
- Molecules of a substance are in a constant motion.
- Matter can be converted into any other state by changing factors like temperature and pressure.























Comments