Carbon is one of the most dynamic elements which are present in the earth. Due to its property of infinite catenation, it can form different types of compounds and has the vastest range of compounds which are known as organic compounds.
Bonding with Carbon-Covalent Bond
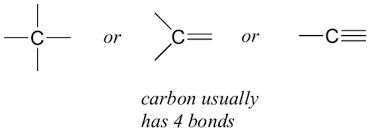
Elements forming ionic compounds achieve this by either gaining or losing electrons from the outermost shell. In the case of carbon, it has four electrons in its outermost shell and needs to gain or lose four electrons to attain noble gas configuration. If it were to gain or lose electrons :
(i) It could gain four electrons forming C4– anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
(ii) It could lose four electrons forming a C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. Not just carbon, but many other elements form molecules by sharing electrons in this manner. The shared electrons ‘belong’ to the outer shells of both the atoms and lead to both atoms attaining the noble gas configuration. Before going on to compounds of carbon, let us look at some simple molecules formed by the sharing of valence electrons.
The simplest molecule formed in this manner is that of hydrogen. As you have learned earlier, the atomic number of hydrogen is 1. Hence hydrogen has one electron in its K shell and it requires one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a molecule of hydrogen, H2. This allows each hydrogen atom to attain the electronic configuration of the nearest noble gas, helium, which has two electrons in its K shell.
Allotropes of Carbon

The element carbon occurs in different forms in nature with widely varying physical properties. Both diamond and graphite are formed by carbon atoms; the difference lies in the manner in which the carbon atoms are bonded to one another. In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other. These two different structures result in diamond and graphite having very different physical properties even though their chemical properties are the same. Diamond is the hardest substance known while graphite is smooth and slippery. Graphite is also a very good conductor of electricity, unlike other non-metals.
Versatile Nature of Carbon
Two factors noticed in the case of carbon are –
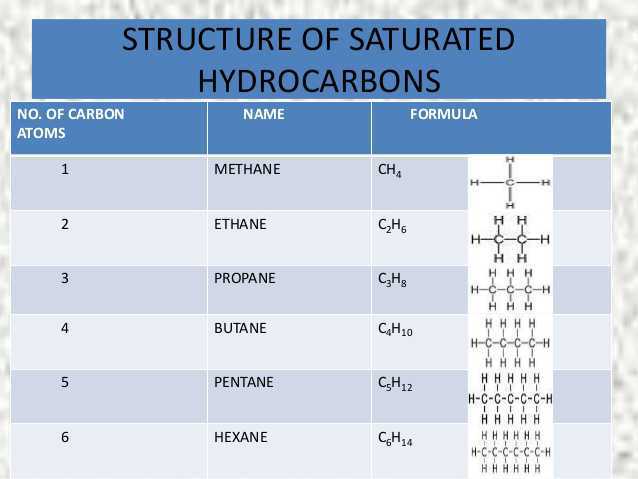
(i) Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings. In addition, carbon atoms may be linked by single, double or triple bonds. Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compounds. Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated compounds.
No other element exhibits the property of catenation to the extent seen in carbon compounds. Silicon forms compounds with hydrogen which have chains of up to seven or eight atoms, but these compounds are very reactive. The carbon-carbon bond is very strong and hence stable. This gives us a large number of compounds with many carbon atoms linked to each other.
(ii) Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties which depend on the elements other than carbon present in the molecule. Again the bonds that carbon forms with most other elements are very strong making these compounds exceptionally stable. One reason for the formation of strong bonds by carbon is its small size. This enables the nucleus to hold on to the shared pairs of electrons strongly. The bonds formed by elements having larger atoms are much weaker.
Recap
- Carbon is a versatile element that forms the basis for all living organisms and many of the things we use.
- This large variety of compounds is formed by carbon because of its tetravalency and the property of catenation that it exhibits.
- Covalent bonds are formed by the sharing of electrons between two atoms so that both can achieve a completely filled outermost shell.
- Carbon forms covalent bonds with itself and other elements such as hydrogen, oxygen, sulphur, nitrogen and chlorine.
- Carbon also forms compounds containing double and triple bonds between carbon atoms. These carbon chains may be in the form of straight chains, branched chains or rings.
- The ability of carbon to form chains gives rise to a homologous series of compounds in which the same functional group is attached to carbon chains of different lengths.
- The functional groups such as alcohols, aldehydes, ketones and carboxylic acids bestow characteristic properties to the carbon compounds that contain them.
- Carbon and its compounds are some of our major sources of fuels.
- Ethanol and ethanoic acid are carbon compounds of importance in our daily lives.
- The action of soaps and detergents is based on the presence of both hydrophobic and hydrophilic groups in the molecule and this helps to emulsify the oily dirt and hence its removal.





























Comments